
New and modern laboratory with the highest quality research equipment and very experienced laboratory specialists.
Research and Development Center works on the Research Agenda. Project: Innovative product development for disinfection with reduction the amount of active substances (compared to products currently available on the market) and effective, including on the bacterial biofilm of antibiotic-resistant bacteria.
Our new Centre includes a virology and microbiology laboratories, we also have our own cell culture laboratory.
The laboratories performs commercial research in the field of testing the effectiveness of disinfectants according to EN 14885:2022-09 “Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics” in in the medical and veterinary area.

OUR LABORATORIES


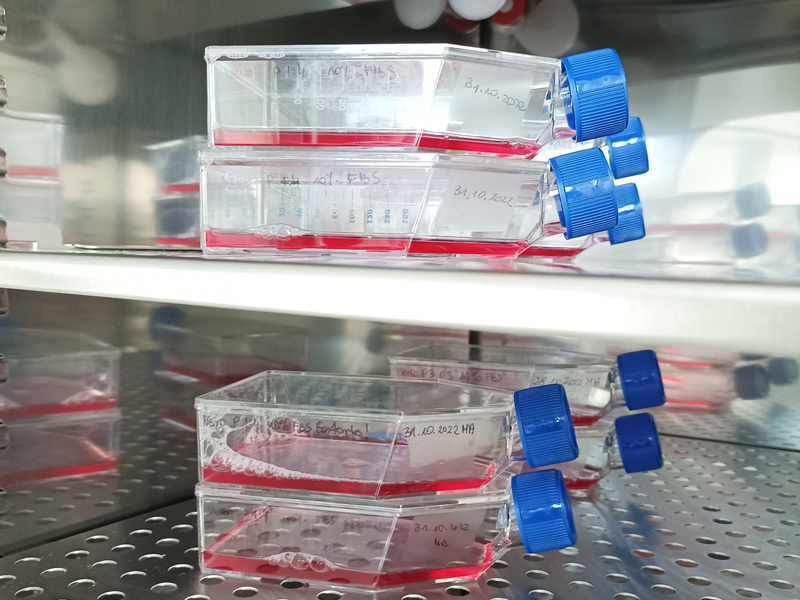
CELL CULTURE LABORATORY
(BIOSAFETY LEVEL 2)
(BIOSAFETY LEVEL 2)
In the BSL Class 2 in vitro cell culture laboratory we culturing of cells derived from mammalian cell lines (Vero, RAW, MDBK), including human cell lines (HeLa).
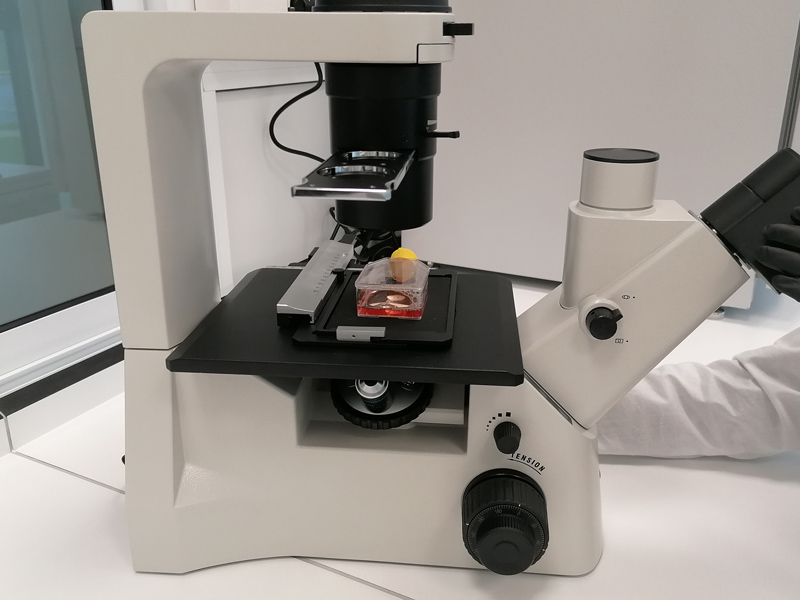
VIROLOGY LABORATORY
(BIOSAFETY LEVEL 2)
(BIOSAFETY LEVEL 2)
Disinfectant Efficacy Testing in accordance with European Standards:
PN-EN 14476:2013+A2:2019 – Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of virucidal activity in the medical area – Test method and requirements (Phase 2, step 1)
EN 17111:2018-Chemical disinfectants and antiseptics -Quantitative carrier test for the evaluation of virucidal activity for instruments used in the medical area (Phase2/step 2)
EN 16777:2018-Chemical disinfectants and antiseptics -Quantitative non-porous surface test without mechanical action for the evaluation of virucidal activity of chemical disinfectants used in the medical area (Phase2/step 2)
PN-EN 14675:2015-06 – Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of virucidal activity of chemical disinfectants and antiseptics used in the veterinary area (Phase 2/step 1)
EN 17122:2020-04-Chemical disinfectants and antiseptics -Quantitative non-porous surface test for the evaluation of virucidal activity of chemical disinfectants and antiseptics used in the veterinary area (Phase 2/step 2)
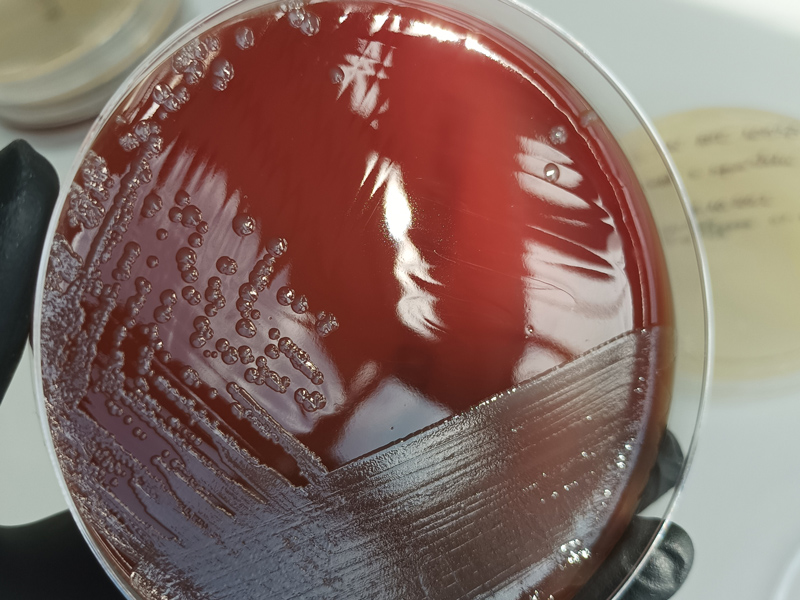
MICROBIOLOGY LABORATORY
(BIOSAFETY LEVEL 2)
(BIOSAFETY LEVEL 2)
Disinfectant Efficacy Testing in accordance with European Standards:
PN-EN 13624:2022-04 – Chemical disinfectants and antiseptics — Quantitative suspension method for determining fungicidal or yeast-killing effects in the medical field — Test method and requirements (Phase 2, step 1)
PN-EN 13727+A2:2015-12 – Chemical disinfectants and antiseptics -Quantitative suspension test for the evaluation of bactericidal activity in the medical area -Test method and requirements (Phase 2, step 1)
PN-EN 13697+A1:2019-08 – Chemical disinfectants and antiseptics – Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas – Test method and requirements without mechanical action (Phase 2, step 2)
EN 1656 -Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in the veterinary area (Phase 2/step 1)
EN 1657 -Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of fungicidal or yeasticidalactivity of chemical disinfectants and antiseptics used in the veterinary area (Phase 2/step 1)
EN 14349 -Quantitative surface test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in the veterinary area on non-porous surfaces without mechanical action (Phase 2/step 2)
EN 16438 -Quantitative surface test for the evaluation of fungicidal or yeasticidalactivity of chemical disinfectants and antiseptics used in the veterinary area on non-porous surfaces without mechanical action (Phase 2/step 2)
CONTACT US
Fill out the form or write to us: cbr@medisept.pl













